
Enormous progress in single-molecule fluorescence microscopy has enabled us to directly observe movements and interactions of individual proteins. Using single-molecule fluorescence microscopy techniques, we are working on dynamics of single molecules and organelles to reveal mysteries of nature. A synergy developed among the different major, including biology, physics and engineering, allow us to study biological questions with the state-of-the-art techniques including real-time three-dimensional nanometer-accuracy tracking setup, Fluorescence resonance energy transfer(FRET) and magnetic tweezers.
Our main research goals are
(1) developing real-time three-dimensional nanometer-accuracy FRET microscopy and
(2) discovering mechanisms of neuronal death in mouse models of neurodegenerative diseases.
(3) investigating exocytosis and endocytosis of inhibitory synaptic vesicles.
(4) investigating the stepping mechanism of myosin X.

I. Developing real-time three-dimensional nanometer-accuracy FRET microscopy
Many questions in biology are about "location" and "interaction" of proteins. For example, how vesicular and membrane proteins interact to enable vesicle docking and priming is basically a question about where proteins are located and when they interact. Simultaneously observing both interaction and location with an accuracy of nanometers would be enormously useful for investigating such questions, but such a technique has not yet been developed
We are developing a microscopy technique for locating the 3D position of organelles or proteins with an accuracy of nanometers and detecting their interactions at the same time. Once we develop this technique, we will apply it to investigate how vesicles undergo docking and priming steps via interactions with active zone proteins such as RIM and Munc13. In addition, this technique can be used for examining gating mechanisms of the store-operated calcium channel Orai. In order to open Orai, the endoplasmic reticulum stromal interaction molecule (STIM) is believed to move and bind Orai. Owing to technical difficulty, there is currently no simultaneous observation of these movements and interactions. We will measure how far STIM moves upon the depletion of internal calcium stores. We will investigate whether a two-part diffusion trap exists and how many STIM molecules are needed to trap Orai.
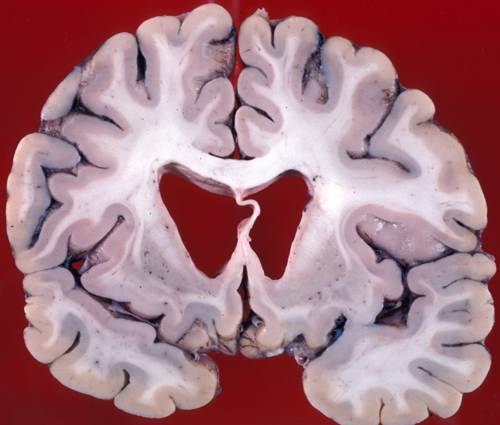
Atrophy of the caudate nuclei and dilatation of the lateral ventricles in Huntington's Disease.
II. Discovering mechanisms of neuronal death in mouse models of neurodegenerative diseases
As people live longer, more people suffer from neurodegenerative diseases. For example, more than 5 millions of people in US are suffering from Alzheimer's disease and the number of Alzheimer's patients will increase to 14 million by 2050. However, there is no currently available treatment and their causes have not been clearly understood yet. Neurodegenerative diseases share pathogenetic mechanisms involving deposition and aggregation of disease-specific proteins, and eventual neuronal death. In order to find therapeutic targets for these neurodegenerative diseases, we have to find how neurons die in those diseases. In order to answer how neurons die in neurodegenerative disease, we will use a model system of Huntington's disease (HD) because HD is a purely genetic disease and its pathogenesis is better understood than other neurodegenerative diseases. HD is caused by expanded CAG units (>35 repeated units) in the huntingtin gene, which are translated into a polyglutamine tract. The mutant huntingtin proteins cause significant dysfunction in neurons, leading to progressive neuronal loss initially in the striatum and then in other regions of the brain. The cause of neuronal loss remains unknown yet. Furthermore, no research has been performed at the single-vesicle level to uncover mechanisms of neuronal death yet.
Using real-time 3D nanometer-accuracy microscopy, we are investigating mechanisms of neuronal death in the striatal neurons from the Q175 knock-in mouse model of HD at the single-vesicle level. We are examining whether disruption of axonal transport and synaptic transmission of synaptic vesicles occurs. In addition, we are investigating the transport of BDNF-containing vesicles and its receptor (TrkB) in cerebral cortical neurons projecting to striatal neurons. This research can be extended to other neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease and familial amyotrophic lateral sclerosis (ALS), which have similar pathogenesis.